New
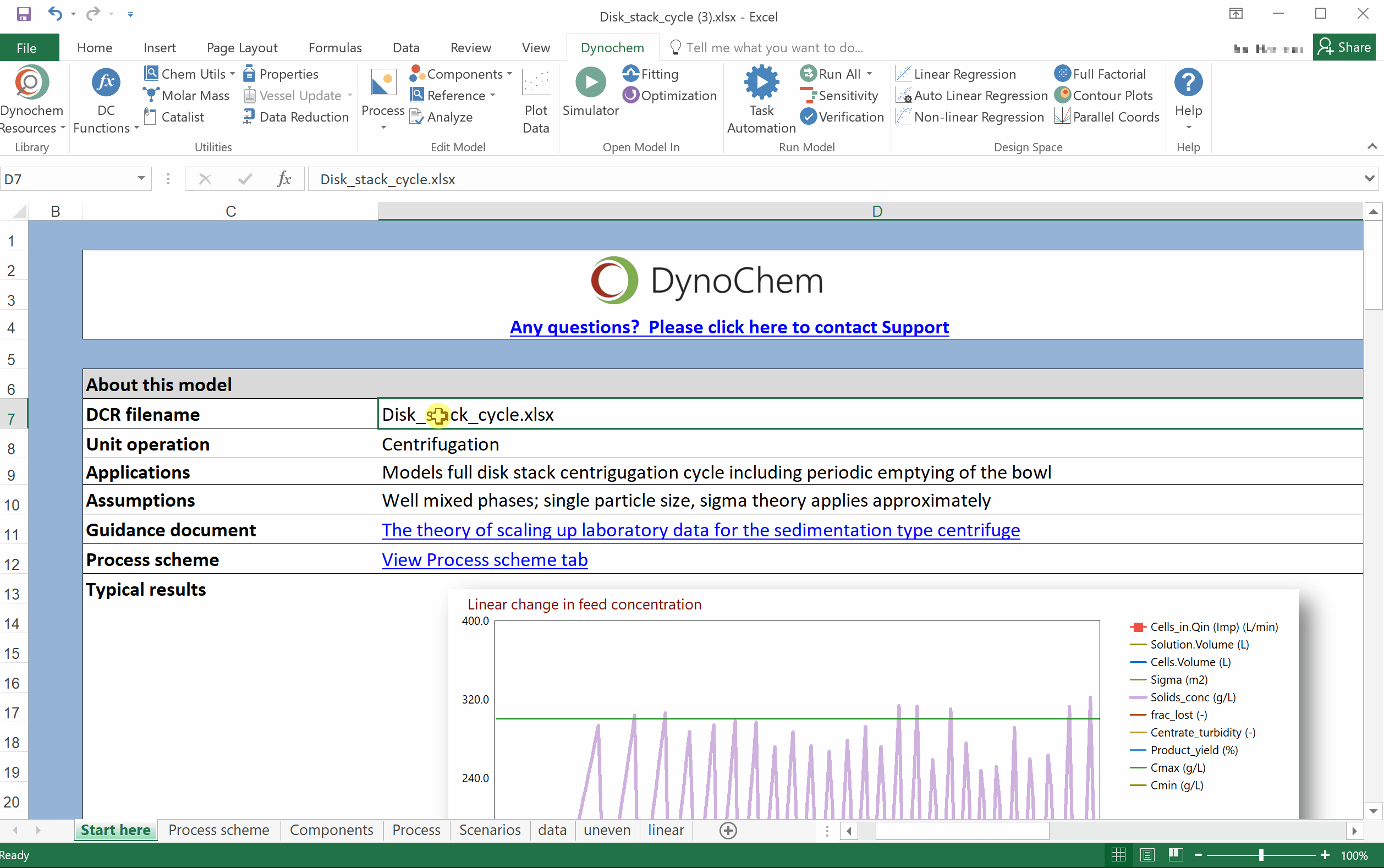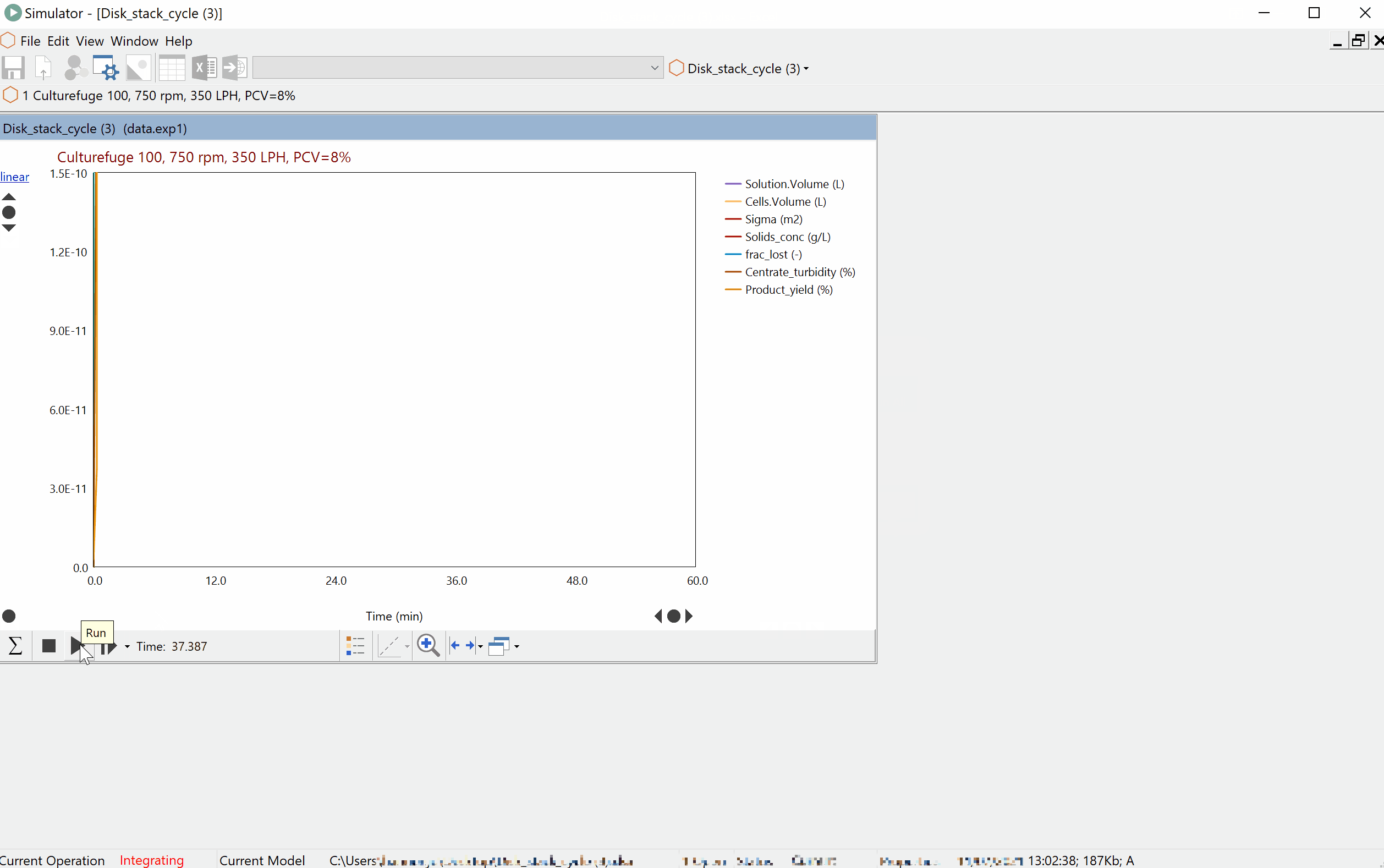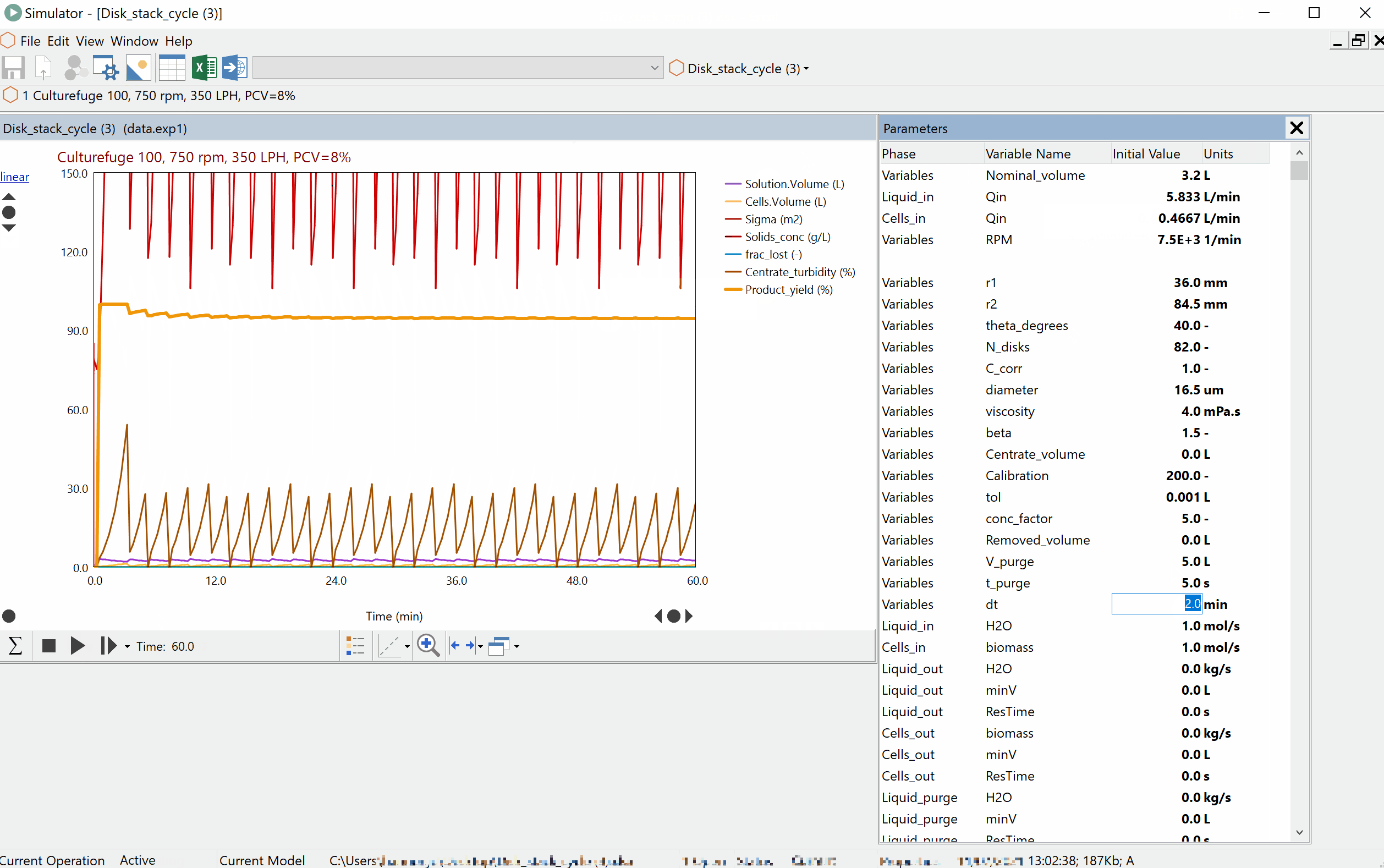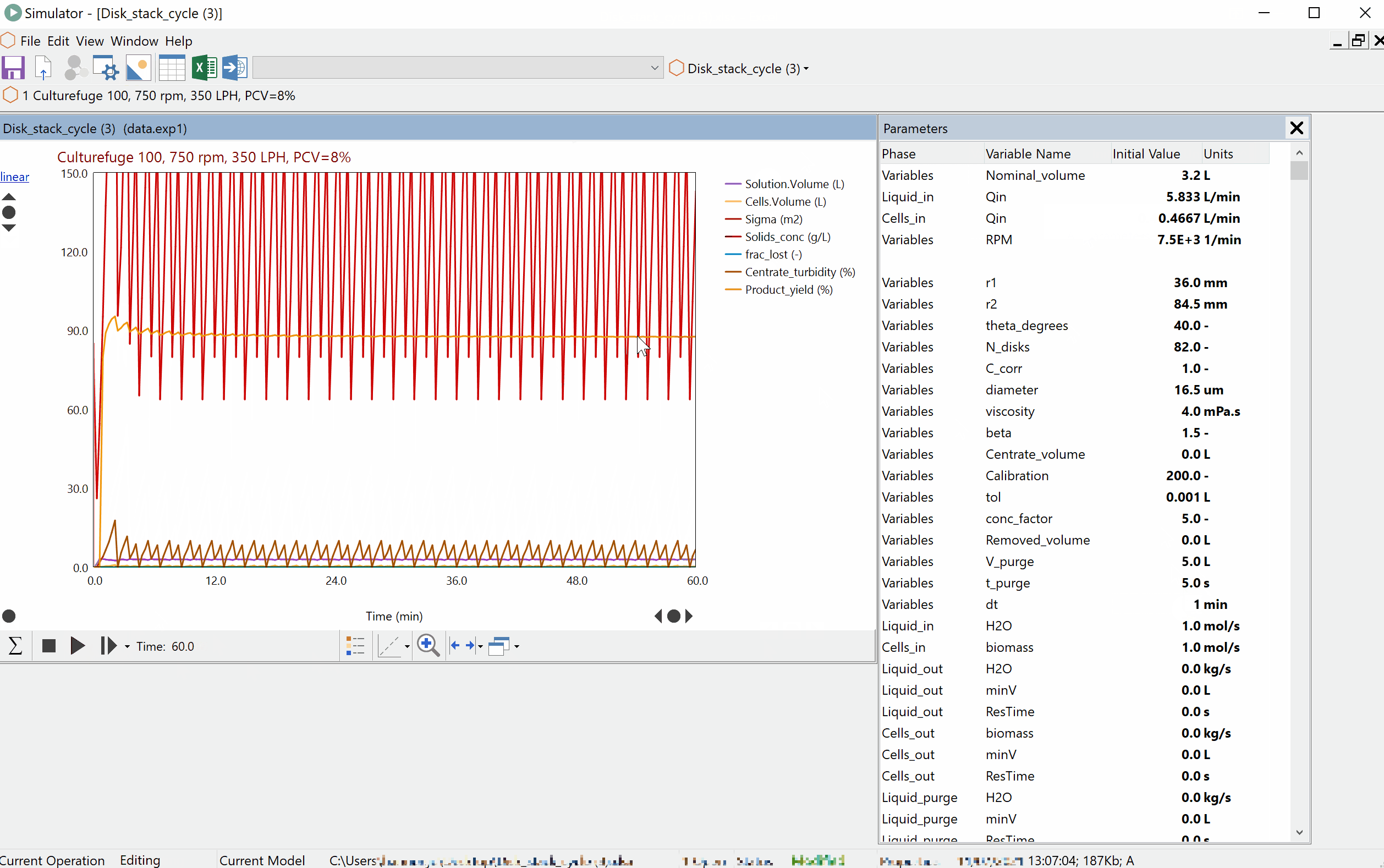
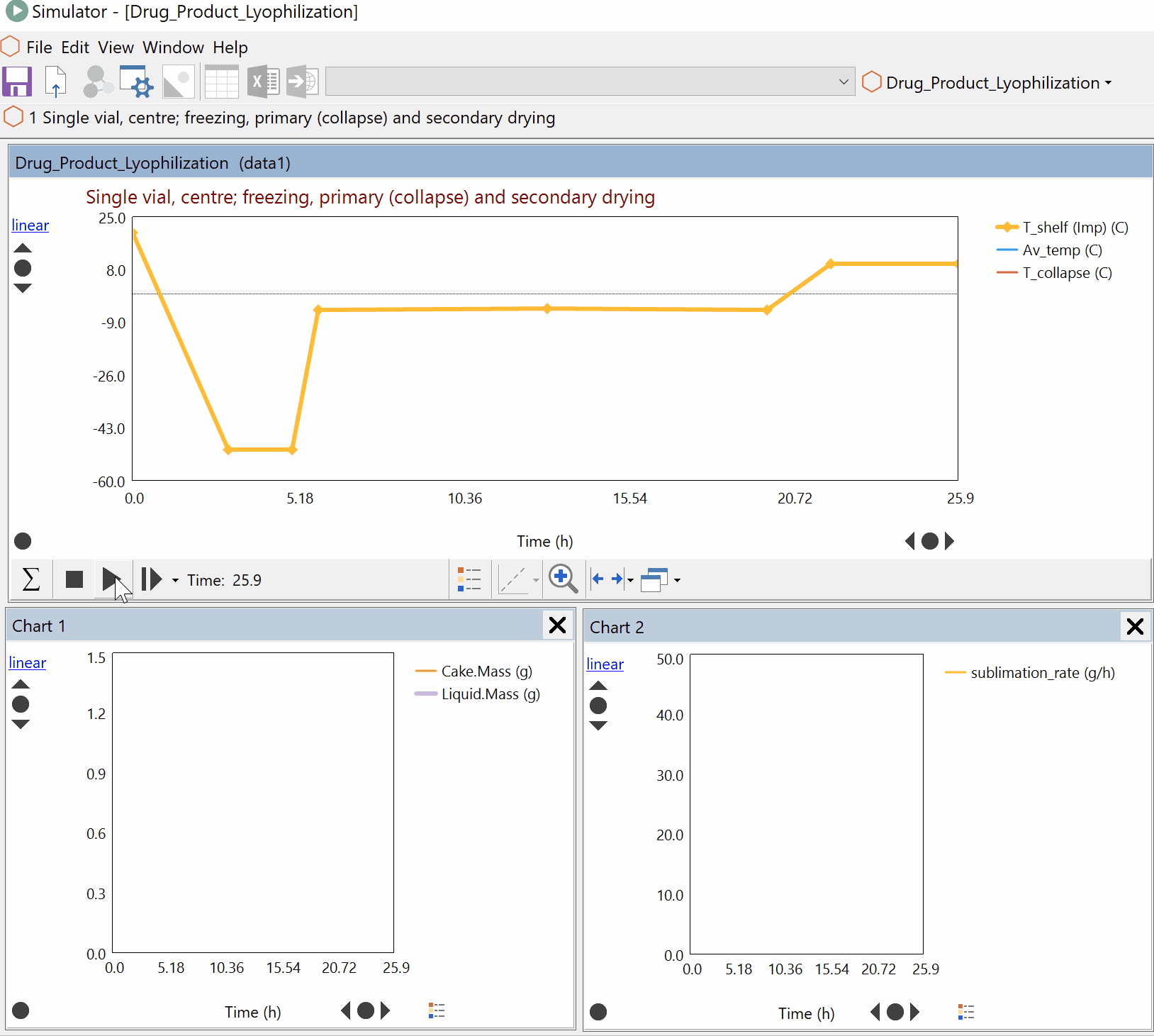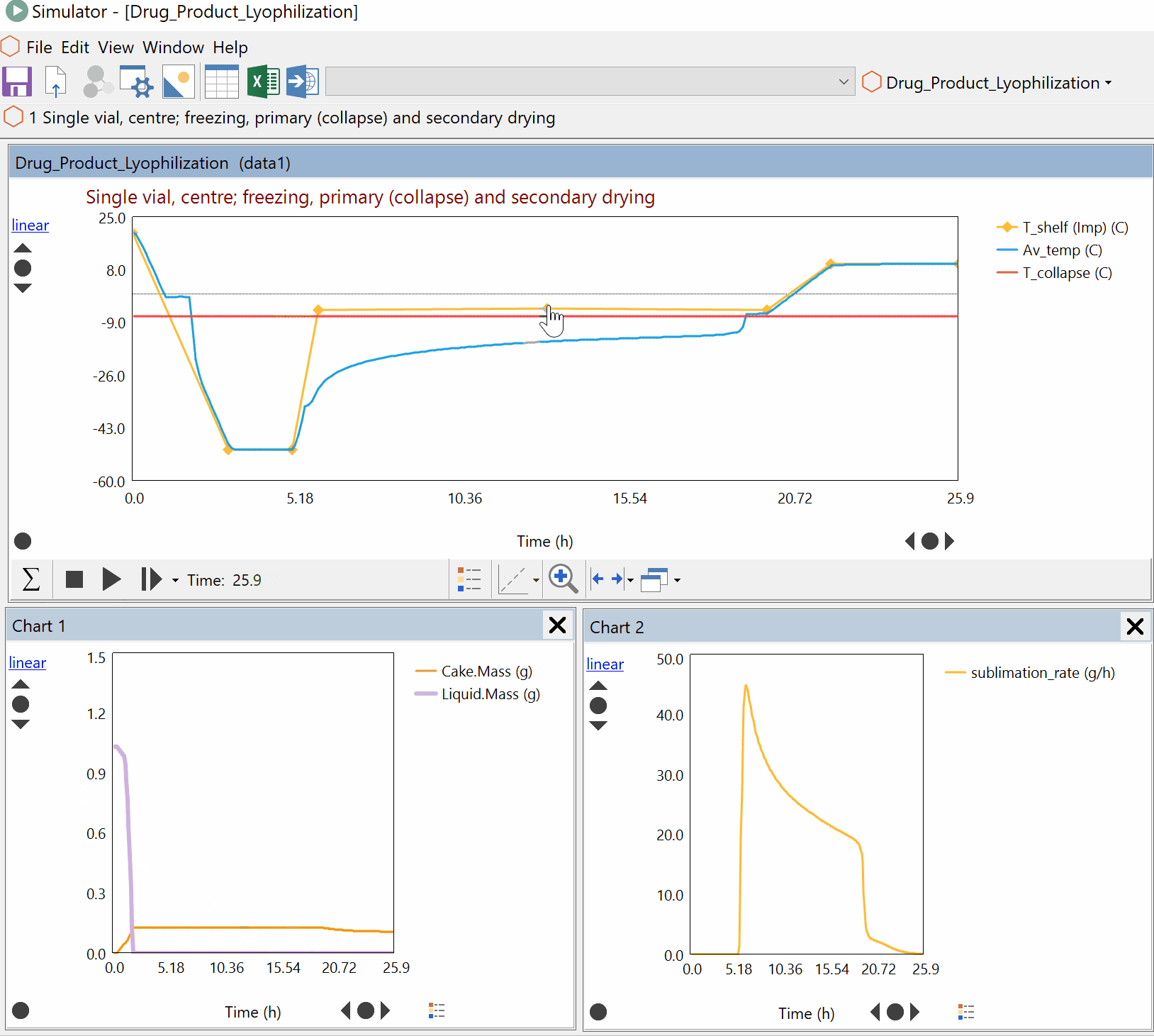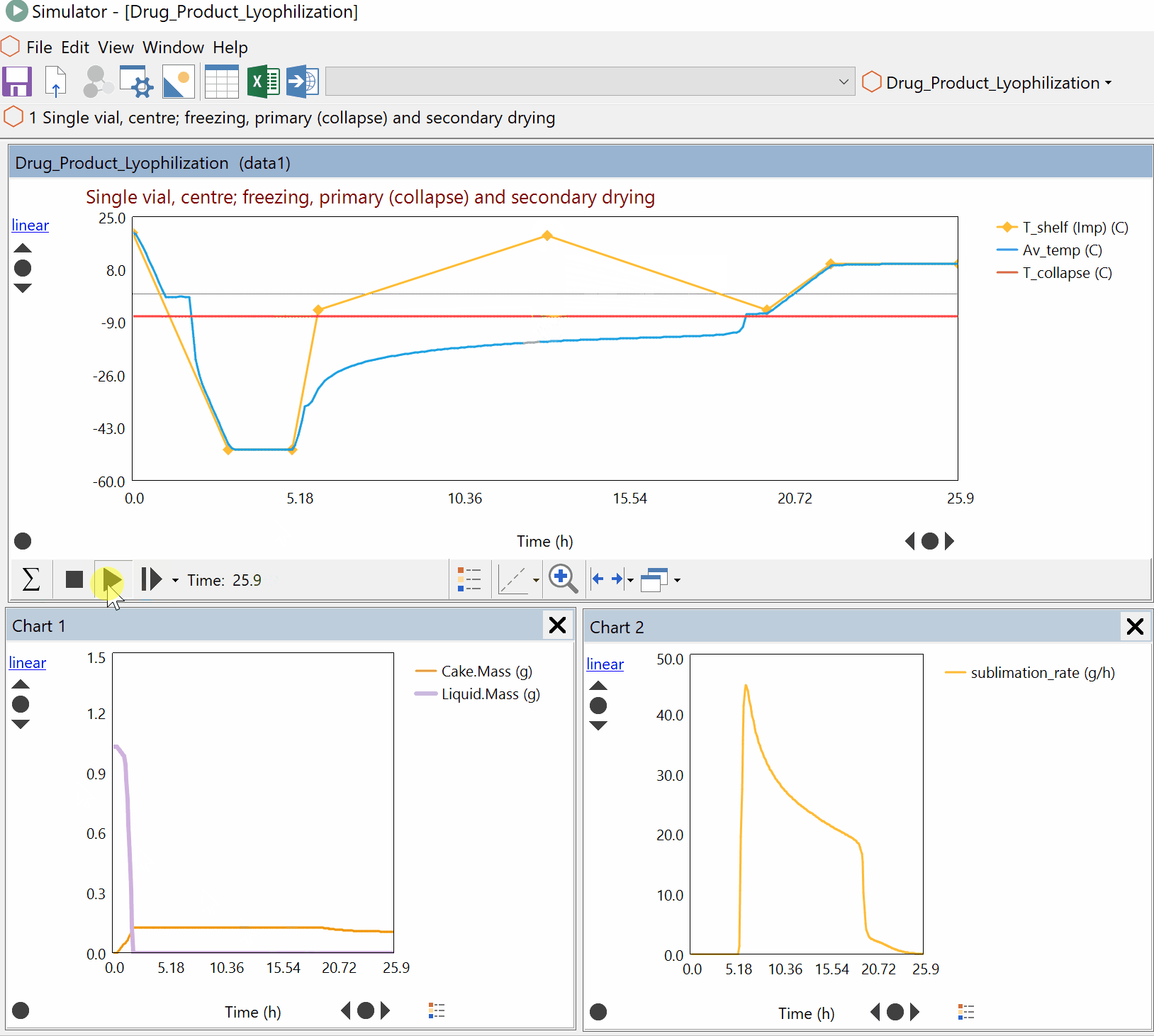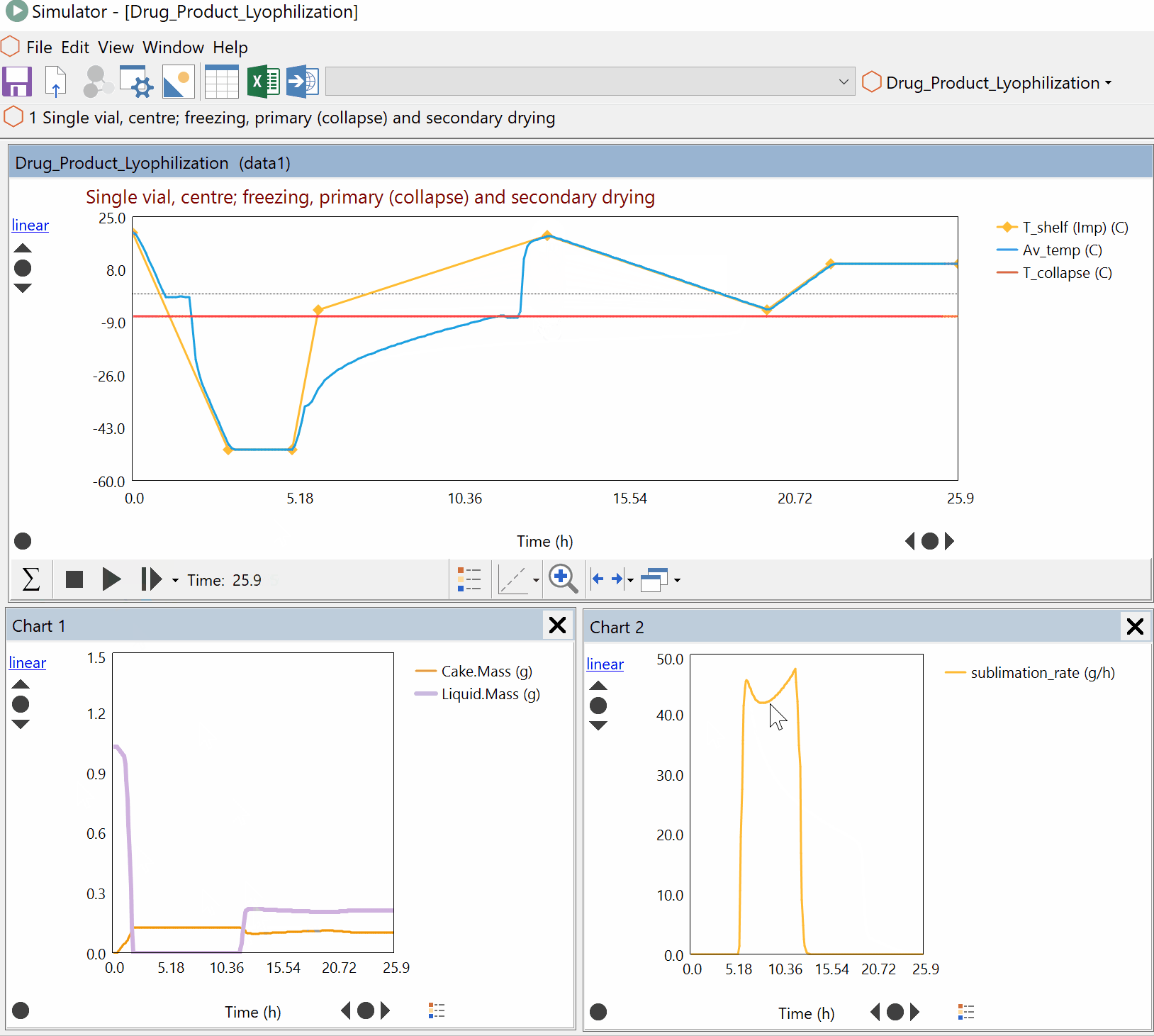
Continuous Reduction of an Ester to Aldehyde in CSTR: From Laboratory to Industrial Plant
Dynochem modelling was used to determine mixing capabilities of different impellers for a continuous DIBAL-H reduction, and the results were used to select the optimum impeller for this system.
2026-Jan-07
Selected highlights at AIChE Annual Meeting 2025
Links to abstracts of presentations featuring use of Scale-up Suite and Mettler Toledo Technologies by Alkermes, BMS, GSK, Genentech, Georgia Tech, Hovione, Lilly, Matheus, MSD (Merck), Sanofi, Takeda, Vertex, Zoetis
2025-Oct-20
Scalable Membrane Enabled One-Pot Liquid-Phase Oligonucleotide Synthesis
This paper describes a one-pot liquid-phase oligonucleotide synthesis enabled by an organic solvent resistant ceramic membrane. Dynochem was used to predict the removal of methanol solvent post-diafiltration (open access paper).
2025-Aug-18
Scalable process development for rAAV transient transfection production using computational fluid dynamics modeling
Recombinant adeno-associated virus (rAAV) is a promising delivery vehicle for cell and gene therapies. Upstream development faces challenges like low productivity and inconsistent performance despite advancements.
2025-Apr-04
Jale Muslehiddinoglu, ST Pharm: Advancing Oligonucleotide Process Development: Integrating First Principles
Improving understanding of processes offers dual benefits for oligonucleotide development. Firstly, it establishes a potential platform for creating similar molecules with different sequences, thereby accelerating process development time /efforts.
2024-Nov-13
Selected highlights at AIChE Annual Meeting 2024
Links to abstracts of presentations featuring use of Scale-up Suite and Mettler Toledo Technologies by AbbVie, Amgen, APC, Boehringer, BMS, GSK, Hovione, Lilly, MSD (Merck), Purdue, Takeda, US Army, Vertex
2024-Oct-15
Let's bring your data up to standard
Outlining the opportunity to use S88 to capture and share development recipes and results of experiments, so that we can move away from transcription errors and create a digital thread that accelerates modeling and tech transfer.
2024-May-31
Selective Reduction of Cysteine Mutant Antibodies for Site-Specific Antibody–Drug Conjugates
Developing site-specific conjugation technologies for antibody–drug conjugates (ADCs) aims to produce more homogeneous and controlled drug-loaded ADCs to reduce variability and thereby improve the therapeutic index.
2023-Nov-20
Selected highlights at AIChE Annual Meeting 2023
Links to abstracts of presentations featuring use of Scale-up Suite by Amgen, BMS, Hovione, Lilly, MSD (Merck), Sarafinas, Seagen, Snapdragon (Cambrex), Veranova.
2023-Oct-27
Kinetic Studies of the Partial Reduction and Conjugation Reactions in an Antibody-Drug Conjugate (ADC) Synthesis
Partially reducing native interchain disulfide bonds of antibodies and covalently conjugating the resulting cysteine thiol groups to potent small-molecule therapeutic agents via linkers result in a heterogeneous mixture of antibody-drug conjugates (ADCs).
2023-Oct-20
Automated End-to-End Workflow for Volumetric Mass-Transfer Coefficient (kLa) Characterization in Small-Molecule Pharmaceutical Development
Biocatalytic aerobic oxidations have recently emerged in the small-molecule pharmaceutical industry as a selective and green alternative to traditional chemocatalyzed oxidations that rely on transition-metal-based oxidants.
2023-Oct-19
Mechanistic Modeling of Tangential Flow Filtration to Guide Process Development
Ultrafiltration (UF) and Diafiltration (DF) are key unit operations for bioprocess development that use tangential flow filtration (TFF) to provide a fast, efficient, and economical means of concentrating and buffer exchanging proteins.
2022-Nov-17
Selected highlights at AIChE Annual Meeting 2022
Links to abstracts of presentations featuring use of Scale-up Suite by BMS, Continuus, Gilead, GSK, Lilly, Merck, On Demand Pharma, Sanofi, Teich Process Development, Vertex, US Army
2022-Nov-03
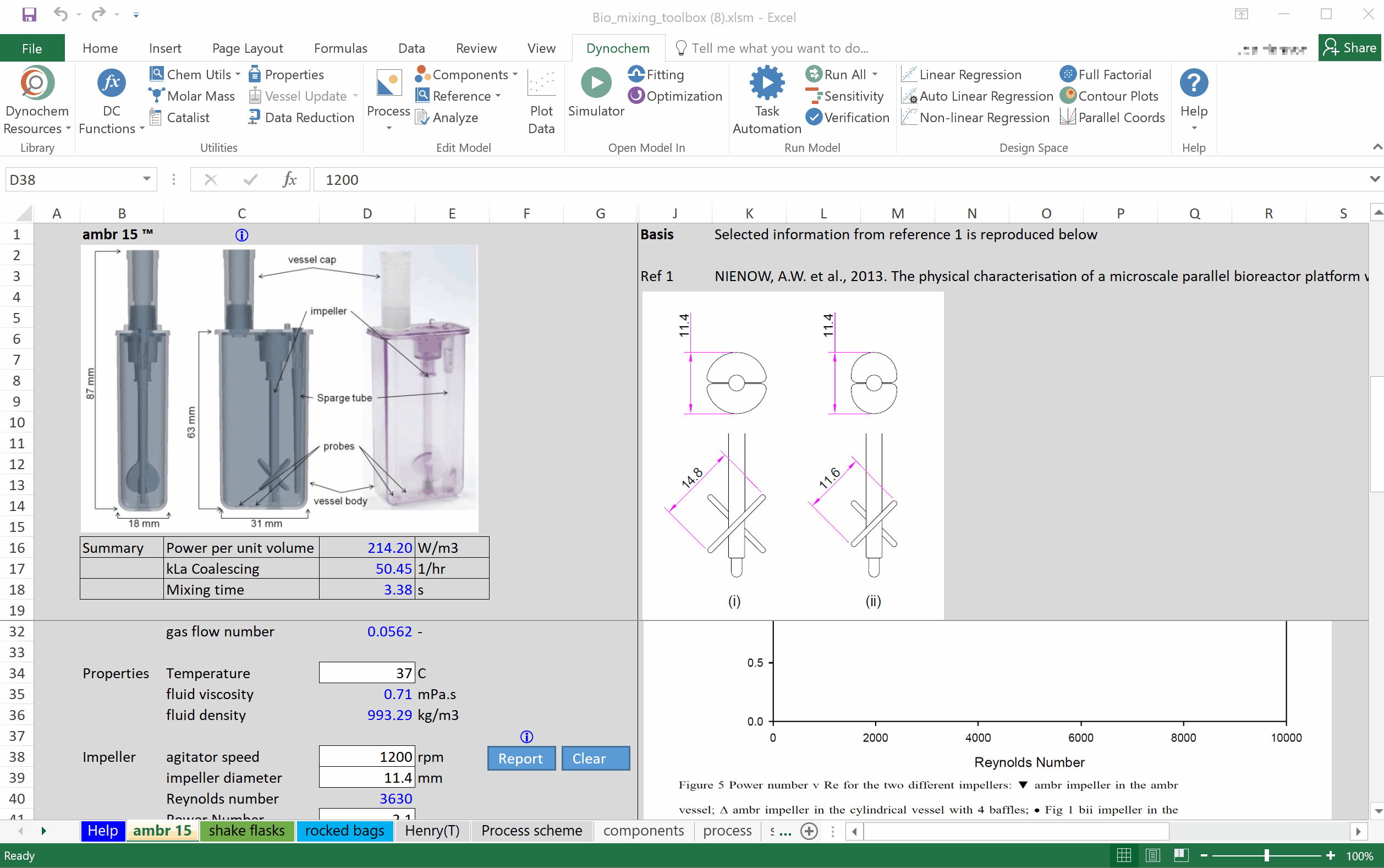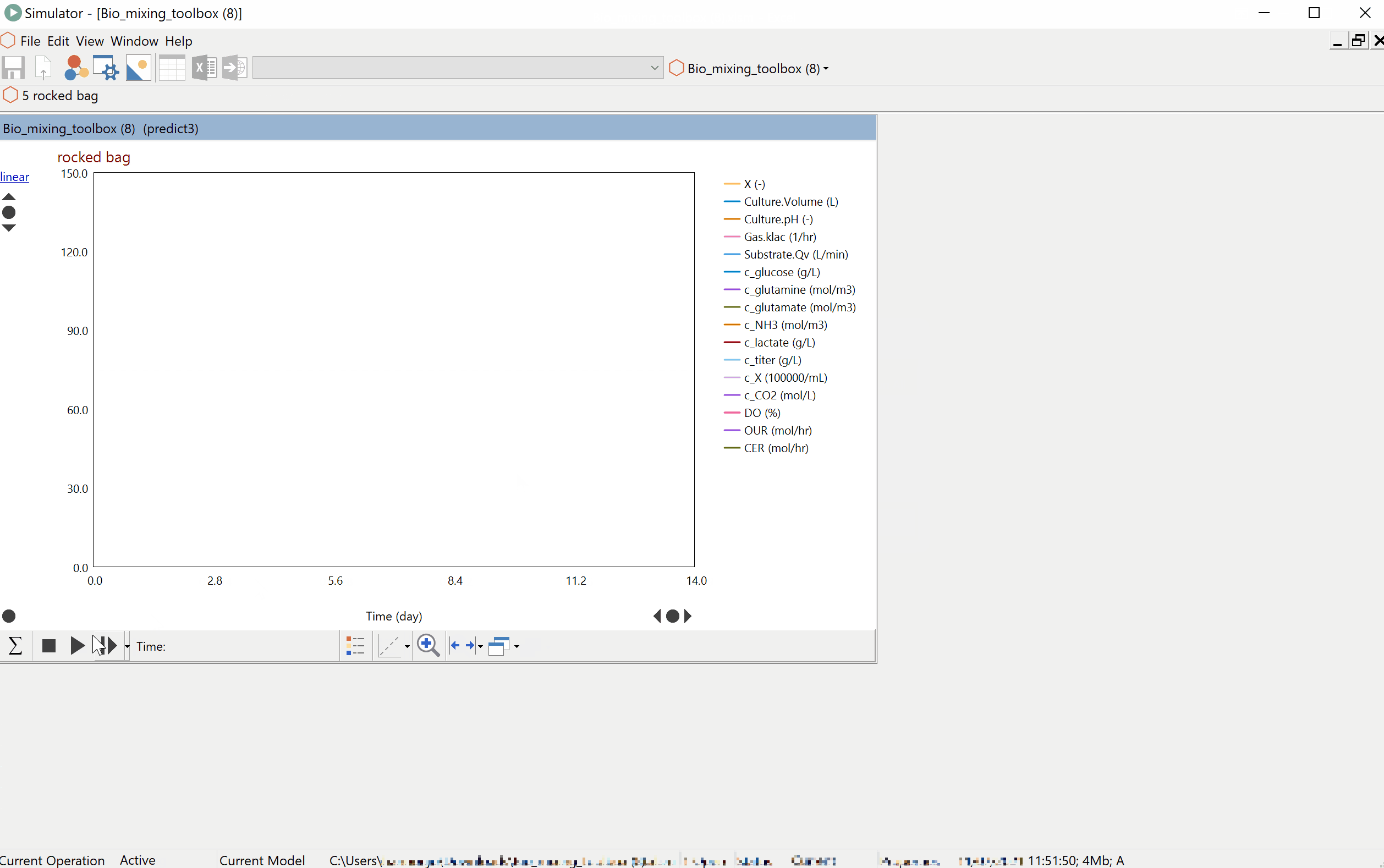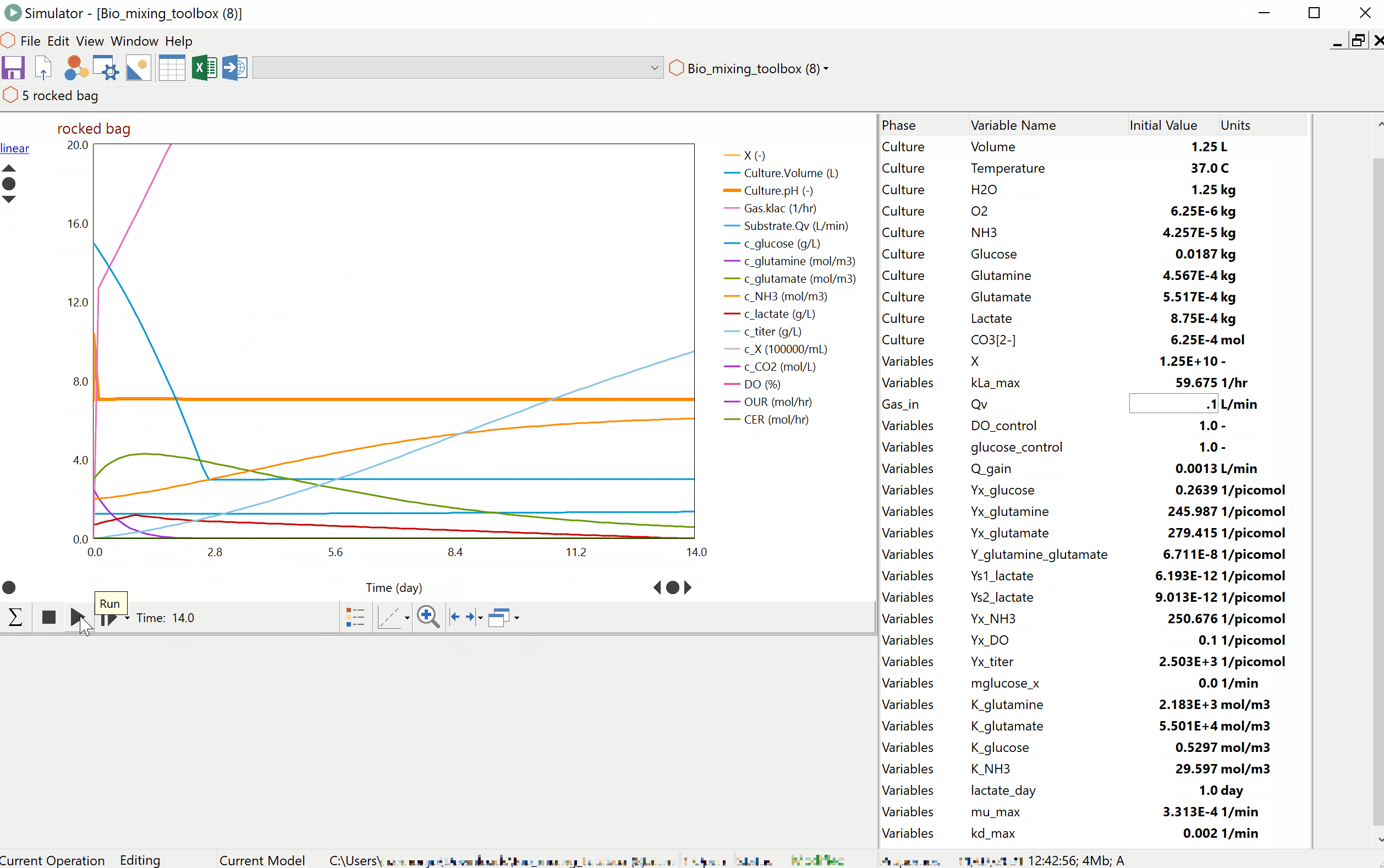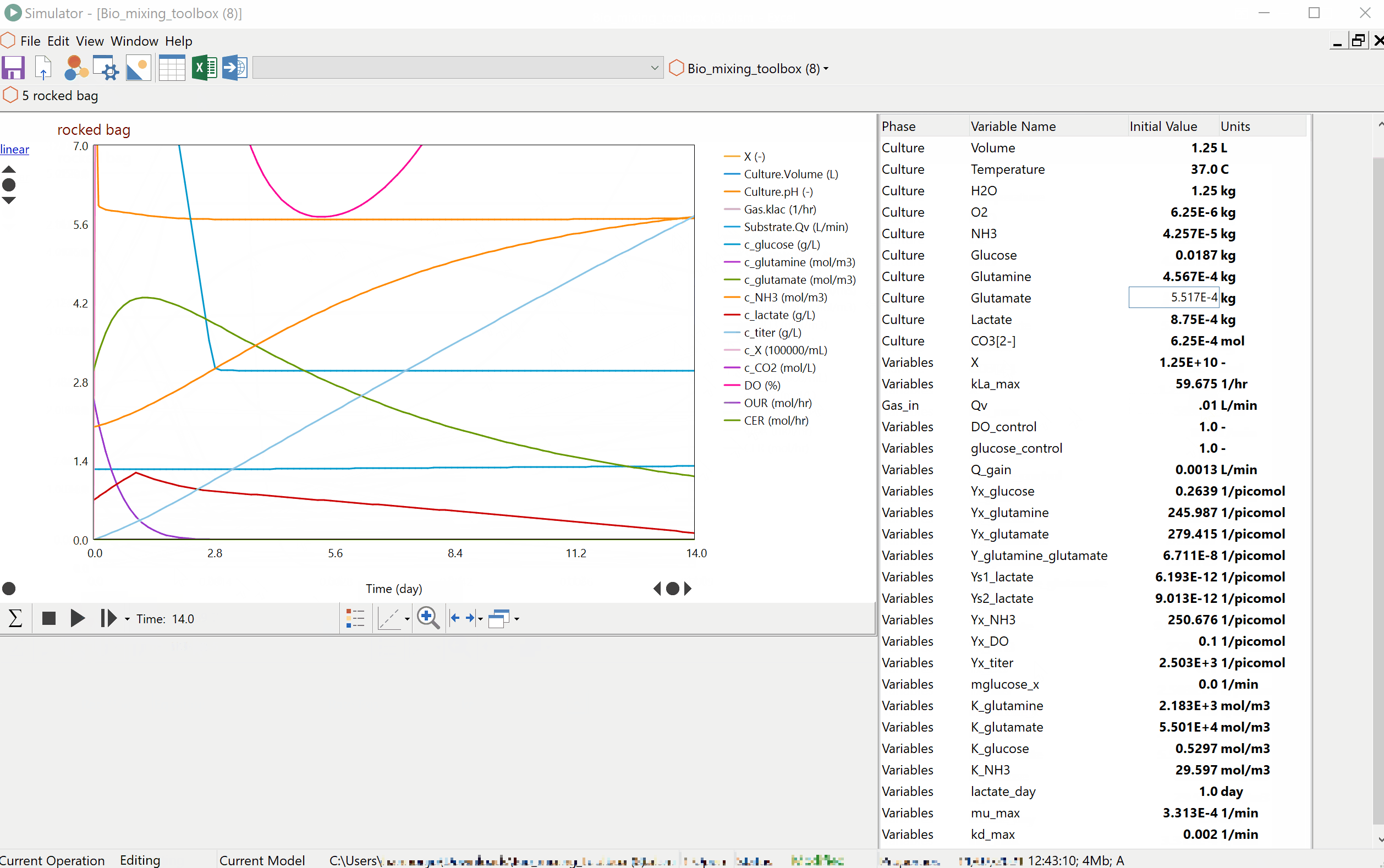
Prof Alvin Nienow on Biologics: Effect of Mixing on Stem Cells, T-Cells and CAR-T Cells
Special features of: a) stem cell culture: microcarrier choice, suspension of microcarriers, cell damage, cell detachment and harvest, bead-to-bead cell transfer; b) T-cells and CAR-T cells, suspension of Dynabeads, use of ambr 250. [Part 3 of 3].
2022-Feb-03
Selected highlights at AIChE Annual Meeting 2021
Links to abstracts of presentations featuring use of Dynochem or Reaction Lab by AbbVie, BMS, Hovione, Lilly, Merck, Sarafinas Process & Mixing Consulting.
2021-Nov-03
Prof Alvin Nienow on Biologics: Effect of Mixing on Animal Cell Culture
Agitation aspects that relate to free suspension animal cell culture; cell damage and loss of productivity, damage from sparged aeration and the use of Pluronic, oxygen transfer and CO2 stripping, single use bioreactors, ambr 15, scale-up. [Part 2 of 3].
2021-Oct-04
Prof Alvin Nienow on Biologics: Basic Mixing Concepts in STRs for Biologics Production
Importance of agitation in biologics production: Reynolds number, turbulent flow, Kolmogorov theory, impeller characteristics, fluid dynamic stress, bulk blending, mass transfer, kLa sensitivity, scale-up. [Part 1 of 3].
2021-Sep-14
Guest: Ronald Carrasquillo & Daniel Perkins, BMS: “Efficient Parameter Space Assessment to Enable the Scale Up of Mixing Operations (A picture is still worth 1000 words)”
We present case studies showing how easy to use scripts, which create response surface plots from rigorous Dynochem mixing calculations over the parameter space, drove good scale-up decisions at CMOs and overcame a preference for simple but costly DoEs.
2021-Jun-09
Promoting a Safe Laboratory Environment Using the Reactive Hazard Evaluation and Analysis Compilation Tool
In the past several years, the U.S. Chemical Safety Board has found an increase in the frequency of laboratory accidents and injuries.
2021-Mar-01
Guest: Keith Mattern, Merck: “End-to-End Automation: Characterizing volumetric mass transfer coefficient (kLa) across process scales using Dynochem"
Application of automated platform for experimental execution and model regression of gas-liquid mass transfer for reactor characterization, scale-up of biocatalytic oxidation. Review of Dynochem model development to capture unique system dynamics. mAbs.
2020-Nov-11
Guest: Laszlo Tamas, TEVA "Using DynoChem to support the development of Biotechnology-based semi-synthetic API Production"
Dr Laszlo Tamas will talk about the role of process modelling using DynoChem in his work and will illustrate this with examples from API process development and scale-up, mAbs
2017-Oct-18
Development and Scale-up of a Biocatalytic Process To Form a Chiral Sulfoxide
A Baeyer–Villiger monooxygenase enzyme has been used to manufacture a chiral sulfoxide drug intermediate on a kilogram scale.
2017-Jan-04
The scale-up of continuous biphasic liquid/liquid reactions under super-heating conditions: methodology and reactor design
Biphasic liquid/liquid reactions are commonplace, however their scale-up under super-heating conditions is not.
2016-Sep-02
Guest: Shane Grosser, Merck: Development and Scale up of Gas-Liquid Systems Facilitated through Process Modeling
Case studies using DynoChem to develop and scale up gas-liquid systems: pressure swings to dry and degas solvents; solvent evaporation to reduce reaction by-products; vessel characterization and optimization of a biocatalytic oxidation.
2015-Aug-12
High cell density cultivation of Pseudomonas putida KT2440 using glucose without the need for oxygen enriched air supply
High Cell Density (HCD) cultivation of bacteria is essential for the majority of industrial processes to achieve high volumetric productivity (g L−1 h−1) of a bioproduct of interest.
2014-Nov-19
Fed-batch strategies using butyrate for high cell densitycultivation of Pseudomonas putida and its use as a biocatalyst
A mathematically based fed-batch bioprocess demonstrated
the suitability of using a relatively cheap and renewable
substrate (butyric acid) for Pseudomonas putida CA-3
high cell density cultivation.
2014-Aug-08
Guest: Flavien Susanne, Novartis: Application of QbD in Continuous Processing: Approach Supported by DynoChem Process Simulation
A methodology based on process simulation using DynoChem was used to develop three consecutive continuous transformations, transfer them to kilogram scale facilities and generate the design space based on the QbD ICH Q8 guidelines
2014-Jun-18
Guest: Andrew Derrick, Pfizer: DynoChem applications in a busy commercial plant
Applications: Scale-up of laboratory heat flow data to pilot and production scale to assist validation based on maximum temperature Assumptions: well mixed phases, dosing controlled reaction
2013-Nov-21
Guest: Ed Delaney, Reaction Science: Application of DynoChem in CMC Regulatory Support
Ed shows how DynoChem kinetic models can be used to address concerns related to control of genotoxic impurities (for example sulfonate ester) in Pharmaceutical APIs
2013-Sep-25
Guest: Dan Hallow, BMS: Application of DynoChem Reaction Modeling to Quality by Design
Dan Hallow of BMS describes how DynoChem was used in a QbD approach for commercialization of an API step, especially to control formation of a critical impurity.
2013-Aug-14
Reaction Kinetics of Nucleophilic Substitution in the Synthesis of Moxifloxacin
Describes use of DynoChem alongside experiments at Dr Reddys to identify the possible reaction pathway and the role of base in order to obtain improved yield with significantly reduced levels of impurities.
2013-Feb-21
Implementing Quality by Design in Pharmaceutical Salt Selection: A Modeling Approach to Understanding Disproportionation
A mechanistic model based on thermodynamics was built to predict disproportionation.
2012-Aug-24
Applications in Kilo Lab Flow Chemistry and Scale-up
Includes examples of modeling reactions in a PFR and centrifugation.
2011-May-30
Models, Mass Balance, and Analytical Data
Models, Mass Balance, and Analytical Data
2011-May-30
Process analysis of the conversion of styrene to biomass and medium chain length polyhydroxyalkanoate in a two-phase bioreactor
Process analysis of the conversion of styrene to biomass and medium chain length polyhydroxyalkanoate in a two-phase bioreactor
2011-May-18
An Example of Utilizing Mechanistic and Empirical Modeling in Quality by Design
An Example of Utilizing Mechanistic and Empirical Modeling in Quality by Design
2010-Nov-19
Use of Mechanistic Models to Develop Design Spaces for Drug Substance Production
Peter Clark of Scale-up Systems discussed the use of mechanistic models with explanations and examples selected for pharmaceutical scientists.
2009-Nov-01
DynoChem Applications in Abbott's Process Safety Laboratory
DynoChem Applications in Abbott's Process Safety Laboratory. Calorimetry. ARC, Rc1, Qr, Hydrogenation, UA.
2009-May-01
An Example from GSK's Design for Manufacture Initiative: Use of Dynochem in Conjunction with Lab and Pilot Scale Data to Advance Process Understanding
DynoChem was used to develop a kinetic model for the main and side reactions using mechanistic understanding and lab/pilot scale data and to establish a suitable mechanism for the reaction to enable troubleshooting throughout lifecycle management.
2009-May-01
Pilot Plant Unit Operations Modeling as a Process Scale Tool
Mixing calculations were performed to estimate mass transfer effects for a fit-for-purpose heterogeneous reaction. Lab scale calorimetry data was scaled-up to determine required addition rates. These rates were implemented and resulted in success.
2009-May-01
Roles of mechanistic and empirical modeling / DOE in achieving Quality by Design
Mechanistic models can enhance DoE models, either indirectly or directly by inclusion in the DoE by numerical response. Response models have the potential to be used to generate the
design space predictively - “dynamic design space”.
2009-May-01
How Process Safety and Environmental lab can guide Process Development
Merck Environmental and Process Safety lab organization. Integration of Environment and Safety to Process Development. Three examples of how Environment and Process Safety can guide process development.
2009-May-01
An Example from GSK's Design for Manufacture Initiative: Use of Dynochem In Conjunction with Lab and Pilot Scale Data to Advance Process Understanding
An Example from GSK's Design for Manufacture Initiative: Use of Dynochem In Conjunction with Lab and Pilot Scale Data to Advance Process Understanding
2008-Nov-01
Process Modeling Based Approach towards Quality by Design for An API Synthetic Step
Process Modeling Based Approach towards Quality by Design for An API Synthetic Step
2008-Nov-01
An Application of Reaction Engineering and Modeling In Quality by Design
An Application of Reaction Engineering and Modeling In Quality by Design
2008-Nov-01
Scale-up of Safety Data using Dynochem
Scale-up of Safety Data using Dynochem. Calorimetry. Gas evolution. Qr. Rc1, Omnical, ARC.
2008-Oct-01
Overview of DynoChem for safety applications
Overview of DynoChem for safety applications
2008-Oct-01
Application of Modeling in a Qbd Context
Application of Modeling in a Qbd Context
2007-Nov-01
Potential of S88, Batchml and Other Standards in Streamlining Process Modeling
Potential of S88, Batchml and Other Standards in Streamlining Process Modeling
2007-Nov-01
Using DynoChem to reduce batch cycle time in a gas-liquid reactor and commission a new unit.
Using DynoChem to reduce batch cycle time in a gas-liquid reactor and commission a new unit.
2007-May-01
Facilitating late stage development through integrated process modeling
Facilitating late stage development through integrated process modeling
2007-May-01
Comparative evaluation of commercial software for kinetic parameter estimation.
Comparative evaluation of commercial software for kinetic parameter estimation.
2007-May-01
DynoChem and homogeneous mixing: an example
DynoChem and homogeneous mixing: an example
2007-May-01
DynoChem case studies in the safety lab
DynoChem case studies in the safety lab. Calorimetry. ARC, Rc1, Qr, kinetics.
2007-May-01
Emerging Technologies Supporting Chemical Process R&D and Their Increasing Impact on Productivity in the Pharmaceutical Industry
Review of parallel experiments (DoE), reaction kinetic modelling, kinetic analysis, QbD (design space) and new technologies (e.g. continuous reactions)
2006-May-27